Research
Our current research has three main themes.
Mechanistic understanding of sustainable aqueous batteries
Aqueous batteries emerge as attractive alternatives for large-scale energy storage, offering several advantages, such as non-flammability, cost-effectiveness, and environmental benignity. However, a notable drawback of aqueous electrolytes is the limited voltage of the battery cell. The narrow electrochemical stability window of water (1.23 V) triggers the hydrogen evolution reaction and the oxygen evolution reaction during battery overcharging. Unlike some organic electrolytes, water does not form electrode passivation layers, and rapid battery failure is often observed.
Our research team conducts physicochemical and electrochemical testing to elucidate the working mechanisms of model electrodes, including layered oxide (e.g., vanadium pentoxide, V2O5) and layered sulfide (e.g., titanium disulfide, TiS2), in sustainable aqueous batteries with dilute electrolytes. We are also interested in other aqueous systems, such as Zn, Mn, and Fe.
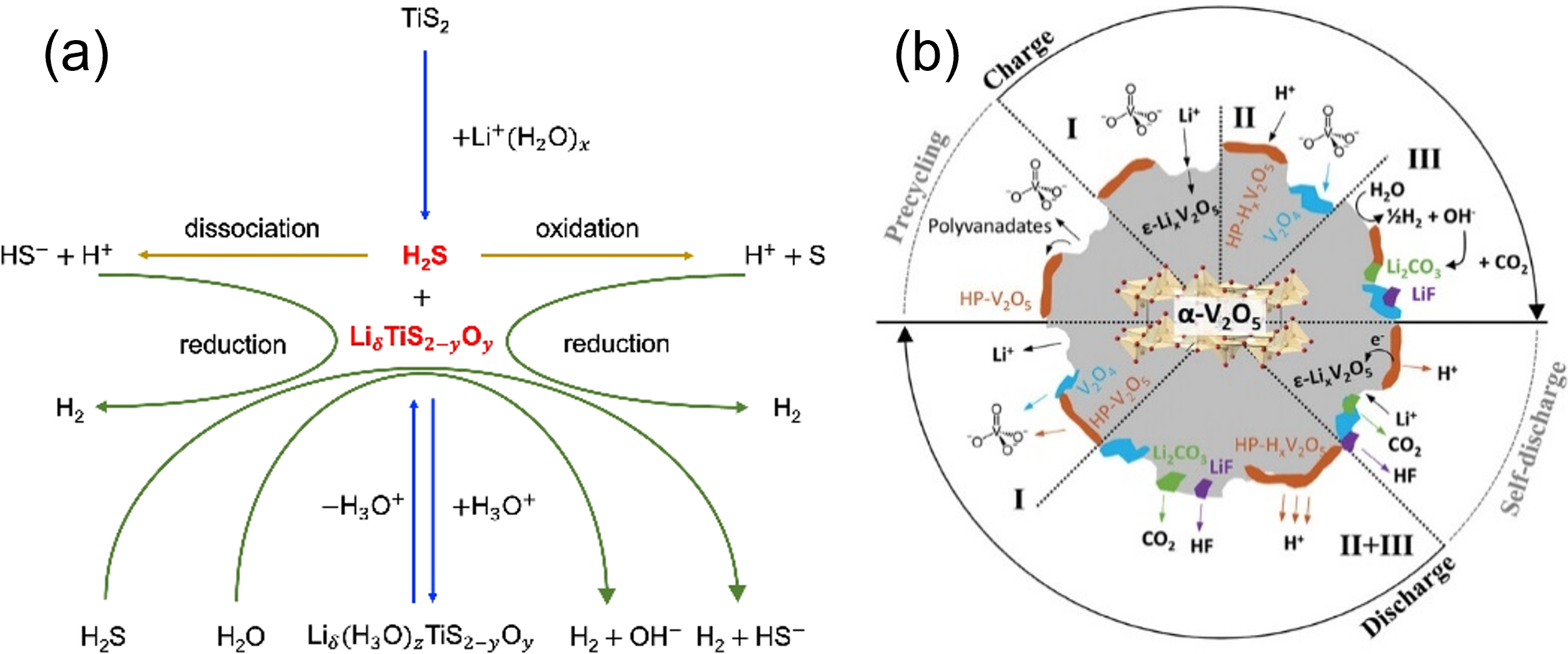
Collaborators: Chao Zhang (Uppsala University), Xu Hou (Lund University), Chaofeng Zhang (Anhui University), and MAX IV Laboratory (Sweden).
References:
- Espinoza Ramos et al. Mater. Today Energy 2025, 51, 101899.
- Li et al. Angew. Chem. Int. Ed. 2025, 64(35), e202508057.
- Zhang et al. Adv. Mater. 2025, 37(13), 2407852.
- Zhang et al. ACS Energy Letter. 2024, 9 (3), 959–966.
- Hou et al. Small 2023, 2308577.
- Zhang et al. Batter. Supercaps 2022, 5 (12), e202200336.
Development and application of advanced operando characterizations
To investigate the battery performance degradation, researchers typically need to stop the cell, extract and prepare electrodes and/or electrolytes for ex situ analysis. However, such conditions typically distort the actual working environment of the sample under study. In contrast, operando techniques allow for continuous analysis without interrupting the cell, providing a more authentic understanding of transient changes occurring inside functioning batteries in real-time.
We possess extensive experience in developing operando techniques, including online acoustic emission testing (AE, for mechanical degradation), online electrochemical mass spectrometry (OEMS, for gas evolution), electrochemical quartz-crystal microbalance with dissipation monitoring (EQCM-D, for solid deposition), and operando pH monitoring (for acid-base reaction).
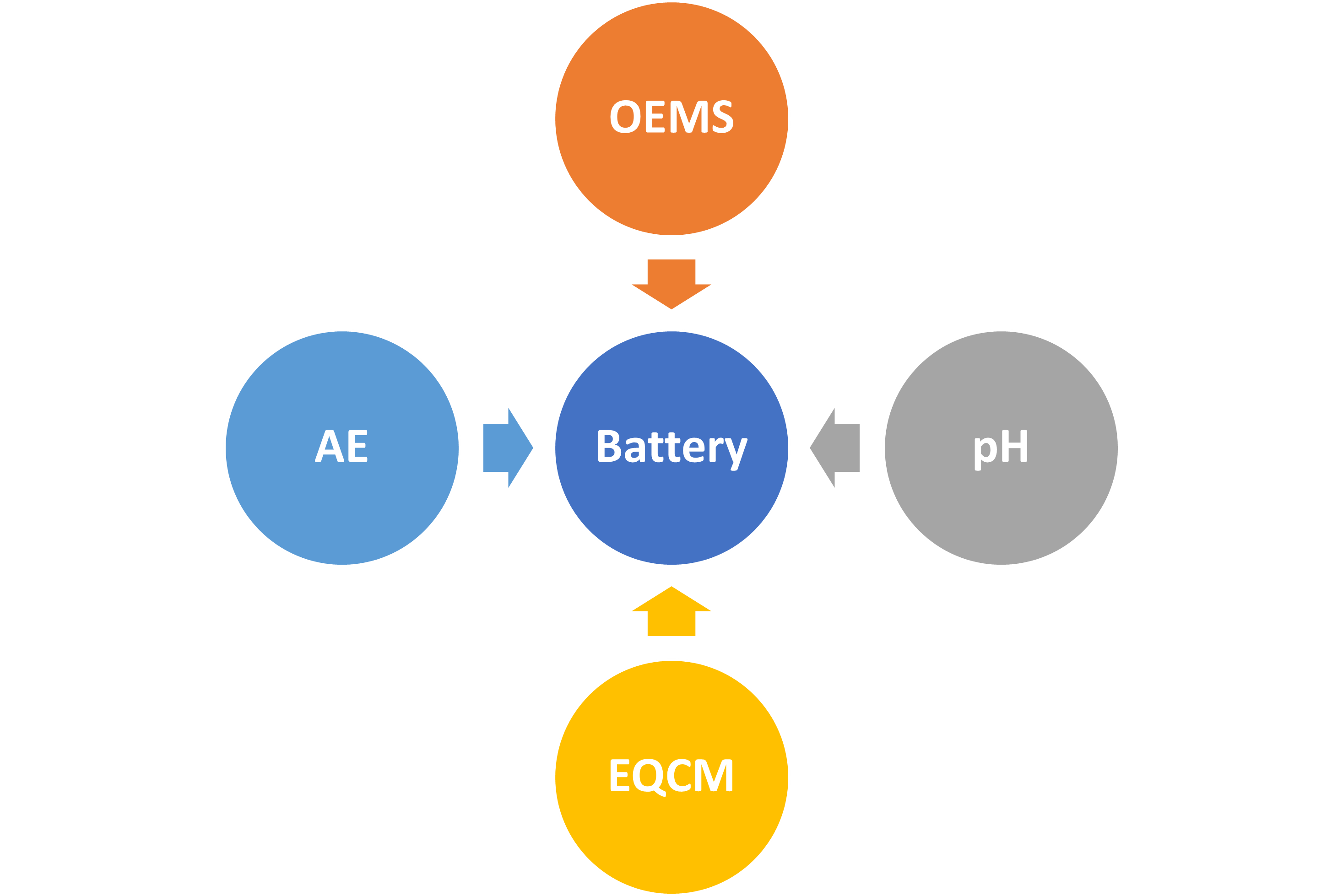
Collaborators: Annika Ahlberg Tidblad (Volvo Cars), Erik Berg (Uppsala University), Jiaqiang Huang (HKUST(GZ)), and Qi Zhao (Hong Kong Polytechnic University).
References:
- Espinoza Ramos et al. Mater. Today Energy 2025, 51, 101899.
- Zheng et al. Energy Environ. Sci 2025, 18, 8499-8514.
- Espinoza Ramos et al. J. Mater. Chem. A 2024, 12 (35), 23280–23296.
- Zhang et al. Energy Storage Mater. 2021, 42, 12–21.
High-throughput robotic screening of battery electrolytes
The integration of data-driven experimentation represents a significant advancement in battery research, substantially expediting progress by closing the experimentation–analysis loop. In contrast to the traditional manual screening of electrodes and electrolytes, automated processes hold the promise of improving both testing efficiency and reproducibility.
At UU, we have pioneered the development of ODACell, an automated platform for electrolyte formulation preparation and coin cell assembly, equipped with three robotic arms and a liquid-handling robot. Our investigations demonstrate that cells assembled by the robots exhibit exceptional reproducibility and consistency. We have also implemented Bayesian optimization to systematically screen additives for high-performance and low-cost aqueous Li-ion batteries using an upgraded workflow ODACell 2.
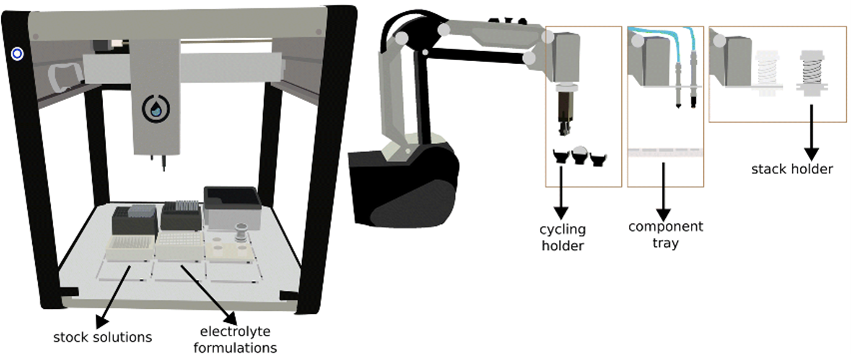
Collaborators: Erik Berg and Jens Sjölund (Uppsala University).
References:
- Yik et al. Cell Rep. Phys. Sci. 2025, 6, 102548.
- Yik et al. Digit. Discov. 2023, 2 (3), 799–808.
